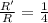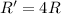In a second-order reaction (one that is second order in one reactant), cutting in half the concentration of that reactant will have what effect on the reaction rate?
a) the reaction rate will remain the same.
b) the reaction rate will double.
c) the reaction rate will decrease by a factor of two.
d) the reaction rate will increase by a factor of four.
e) the reaction rate will decrease by a factor of four.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which of these sequences lists the correct order for the creation of sedimentary rock from sediment? a. deposition, burial, compaction, cementation b. burial, deposition, compaction, cementation c. compaction, deposition, burial, cementation d. cementation, deposition, burial, compaction
Answers: 1

Chemistry, 22.06.2019 02:30
List four observations that indicate that a chemical reaction may be taking place
Answers: 1

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1
You know the right answer?
In a second-order reaction (one that is second order in one reactant), cutting in half the concentra...
Questions

History, 16.05.2021 16:10

Physics, 16.05.2021 16:10


Biology, 16.05.2021 16:10

Biology, 16.05.2021 16:10


Mathematics, 16.05.2021 16:10



Mathematics, 16.05.2021 16:10

Mathematics, 16.05.2021 16:10



Geography, 16.05.2021 16:10


Mathematics, 16.05.2021 16:10


Biology, 16.05.2021 16:10

Biology, 16.05.2021 16:10

Physics, 16.05.2021 16:10

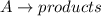
![R=[A]^2](/tpl/images/0501/2093/aeb03.png) (1)
(1)![R'=[\frac{A}{2}]^2](/tpl/images/0501/2093/c4519.png) (2)
(2)