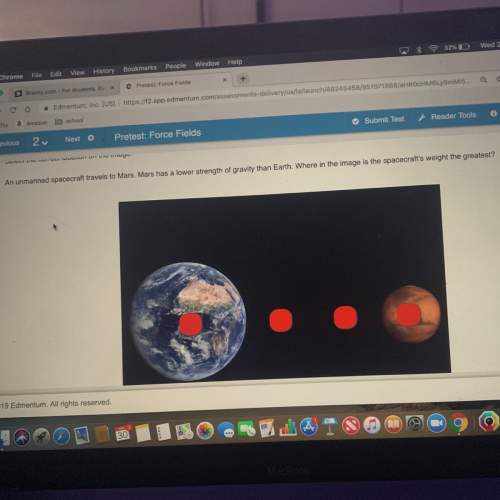
The energy required to separate the ions in a CsF crystal lattice into individual Cs^+1 (g) and F^-1 (g) ions is known as the lattice energy of CsF(s). As shown in the table below, the lattice energy of CsF(s) is smaller than the lattice energy of a similar compound, KF(s). Which of the following best explains why the lattice energy of CsF is smaller than the lattice energy of KF?
CsF = 759
KF = 829
A.) Cs^+ contains more core electrons than K4+, so the valence electrons in Cs^+ are more shielded from the nucleus than the valence electrons in K+.
B.) Cs^+ has a larger ionic radius than K^+, so the distance between cation and anion is greater in CsF than in KF.
C.) Cesium and fluorine have a greater electronegativity difference than potassium and fluorine, so the Cs-F bond is more polar than the K-F bond.
D.) Cesium has a smaller first ionization energy than potassium, so less energy is required to form the Cs^+ ion than to form the K^+ ion.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 14:00
The two naturally occurring isotopes of chlorine are 35cl (34.969 amu, 75.77%) and 37cl (36.966 amu, 24.23%). the two naturally occurring isotopes of bromine are 79br (78.918 rm amu, 50.69%) and 81br (80.916 amu, 49.31%). chlorine and bromine combine to form bromine monochloride, brcl. 1. how many peaks will be present in a mass spectrum for brcl? the four combinations of molecule possible given these four isotopes are: 81br37cl, 81br35cl, 79br37cl, and 79br35cl. 2. what are the masses of the four different brcl molecules? express the masses using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.
Answers: 3

Chemistry, 22.06.2019 15:00
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Chemistry, 23.06.2019 05:00
How many atomic mass units are equal to 1.672×10−24 g of protons?
Answers: 3

Chemistry, 23.06.2019 07:30
Assignment directions: pick one of the following chemists and perform a bit of research on him/her. answer the following questions. alice hamilton rosalind franklin marie curie gertrude b. elion ada yonath henry cavendish robert boyle antoine lavoisier mario j. molina svante arrhenius
Answers: 1
You know the right answer?
The energy required to separate the ions in a CsF crystal lattice into individual Cs^+1 (g) and F^-1...
Questions



Mathematics, 12.02.2021 01:00


Mathematics, 12.02.2021 01:00






Mathematics, 12.02.2021 01:00

Mathematics, 12.02.2021 01:00


Mathematics, 12.02.2021 01:00



Mathematics, 12.02.2021 01:00



History, 12.02.2021 01:00