
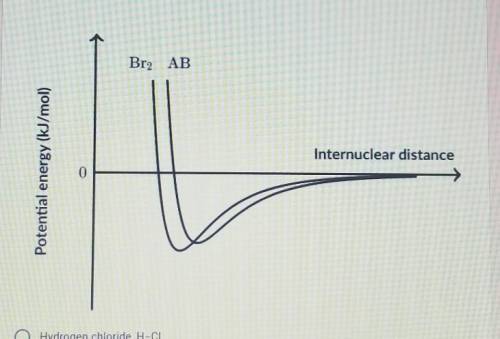
The graph below shows the potential energy as a function of internuclear distance for molecular bromine, Br-Br and an unknown heteronuclear diatomic molecule, A-B. Based on the data in the graph, which of the following correctly identifies the diatomic molecule A-B?
A.) Hydrogen Chloride, H-Cl
B.) Carbon Monoxide, C=_O (Triple Bond)
C.) Sulfur Monoxide, S=O (Double Bond)
D.) Iodine Monobromide, I-Br

Answers: 2
Another question on Chemistry



Chemistry, 22.06.2019 12:30
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3

You know the right answer?
The graph below shows the potential energy as a function of internuclear distance for molecular brom...
Questions



English, 06.11.2020 23:50

Physics, 06.11.2020 23:50


History, 06.11.2020 23:50

Chemistry, 06.11.2020 23:50

Mathematics, 06.11.2020 23:50




Mathematics, 06.11.2020 23:50




Spanish, 06.11.2020 23:50


History, 06.11.2020 23:50




