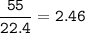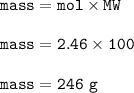Chemistry, 07.12.2020 06:00 nails4life324
When heated, calcium carbonate decomposes to yield calcium oxide and carbon dioxide gas via the reaction
CaCO3(s)→CaO(s)+CO2(g)
What is the mass of calcium carbonate needed to produce 55.0 L of carbon dioxide at STP?
Express your answer with the appropriate units.
mass of CaCO3 =

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1

Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1


Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1
You know the right answer?
When heated, calcium carbonate decomposes to yield calcium oxide and carbon dioxide gas via the reac...
Questions












Computers and Technology, 11.07.2019 06:30


Chemistry, 11.07.2019 06:30

Health, 11.07.2019 06:30


Mathematics, 11.07.2019 06:30

Mathematics, 11.07.2019 06:30


History, 11.07.2019 06:30