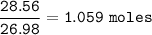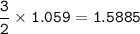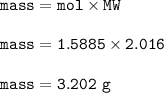Chemistry, 07.12.2020 06:30 natasniebow
How many grams of hydrogen gas (2.016 g/mol) can be produced when 28.56 g of aluminum (26.98 g/mol) are reacted?

Answers: 3
Another question on Chemistry

Chemistry, 23.06.2019 01:00
How does carbon monoxide pose the greatest threat to humans? a. it can be produced by wood fires. b. it can be produced by home furnaces. c. it is produced by acid rain. d. it is produced by modern automobiles.
Answers: 2



Chemistry, 23.06.2019 08:00
Determine the number of moles of air present in 1.35 l at 750 torr and 17.0°c. which equation should you use? n=pv/rt what is the number of moles present? ⇒ 0.056 mol a sample of n2 gas occupying 800.0 ml at 20.0°c is chilled on ice to 0.00°c. if the pressure also drops from 1.50 atm to 1.20 atm, what is the final volume of the gas? which equation should you use? v2= p1v1t2/p2t1 what is the final volume of the gas? ⇒ 932 ml these are the answers
Answers: 1
You know the right answer?
How many grams of hydrogen gas (2.016 g/mol) can be produced when 28.56 g of
aluminum (26.98 g/mol)...
Questions




SAT, 09.02.2021 23:00


Mathematics, 09.02.2021 23:00




Biology, 09.02.2021 23:00





Mathematics, 09.02.2021 23:00

Mathematics, 09.02.2021 23:00

Mathematics, 09.02.2021 23:00

Mathematics, 09.02.2021 23:00

English, 09.02.2021 23:00

Mathematics, 09.02.2021 23:00