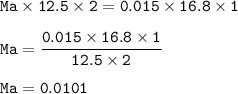Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:20
Which type of substance ionizes partially and gives off hydrogen ions when dissolved in water? a. strong acid b. strong base c. weak acid d. weak base
Answers: 1

Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2

Chemistry, 22.06.2019 21:00
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1

Chemistry, 23.06.2019 03:10
Which of the following compounds would be expected to have the strongest ionic bonds? a)the compound that has b)the largest ions with the greatest charge c)the compound that has d)the largest ions with the least charge the compound that has the smallest ions with the greatest charge the compound that has the smallest ions with the least charge
Answers: 2
You know the right answer?
By titration, 12.5 mL of aqueous H2SO4 neutralized 16.8 mL of 0.015 M LiOH solution.
What was the m...
Questions


English, 08.03.2021 19:40


Biology, 08.03.2021 19:40

English, 08.03.2021 19:40

Mathematics, 08.03.2021 19:40

Physics, 08.03.2021 19:40


History, 08.03.2021 19:40


Mathematics, 08.03.2021 19:40

Mathematics, 08.03.2021 19:40



Mathematics, 08.03.2021 19:40


English, 08.03.2021 19:40

History, 08.03.2021 19:40

Mathematics, 08.03.2021 19:40

Medicine, 08.03.2021 19:40