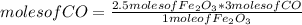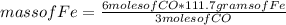Consider the process used to produce iron metal from its ore.
Fe2O3(s) + 3CO(g) --> 2Fe(s) + 3CO2 (g)
How many grams of iron can be produced from 2.5 moles of Fe2O3 and 6.0 moles of CO? Hint: limiting reactant problem
O A. 140 g
B. 335 g
C. 55.858
D. 223 g Fe
E. 279 g Fe

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
This large tectonic plate is bounded on three sides by whats know as the ring of fire. what is the name of this tectonic plate? a) pacific plate b) eurasian plate c) north american plate d) indo- australian plate plz it's science but there's no option for science so i picked chemistry
Answers: 2

Chemistry, 22.06.2019 23:00
In the reaction h2co3 (aq) + 3nh3 (aq) = 2 nh4+ (aq) + co3 2-, how many electrons are transferred?
Answers: 3

Chemistry, 23.06.2019 00:00
Which is true about metals used for jewelry, such as platinum and gold? a. they have low flammability. b. they have low reactivity. c. they have high flammability. d. they have high reactivity.
Answers: 1

You know the right answer?
Consider the process used to produce iron metal from its ore.
Fe2O3(s) + 3CO(g) --> 2Fe(s) + 3CO...
Questions

Mathematics, 13.08.2021 05:50

Mathematics, 13.08.2021 06:00

Mathematics, 13.08.2021 06:00

Mathematics, 13.08.2021 06:00


Mathematics, 13.08.2021 06:00


Mathematics, 13.08.2021 06:00


Social Studies, 13.08.2021 06:00


Mathematics, 13.08.2021 06:00

Mathematics, 13.08.2021 06:00


Mathematics, 13.08.2021 06:00


History, 13.08.2021 06:00

Medicine, 13.08.2021 06:00


Mathematics, 13.08.2021 06:00