
Chemistry, 08.12.2020 05:20 gonzalesrosalinda66
Three possible resonance structures for the fulminate ion, CNO^-1, are
shown below. Based on the formal charges, which of the three structures
contributes most to the resonance hybrid of CNO^-1 ?
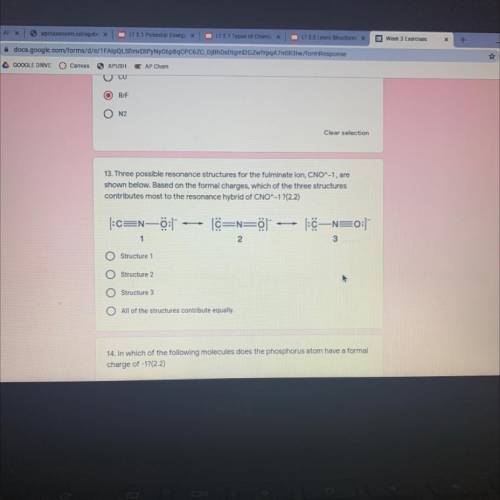

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 16:30
Why should the scientific method be used to answer a question? a. it provides a way to test an idea without any bias. b. it provides a way to test a hypothesis. c. it provides a way to ensure all hypotheses are proven correct. d. it provides a way to quickly turn a hypothesis into a scientific theory.
Answers: 1

Chemistry, 22.06.2019 09:00
Identify the electromagnets with poles that are reversed from the electromagnet shown above
Answers: 3

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1
You know the right answer?
Three possible resonance structures for the fulminate ion, CNO^-1, are
shown below. Based on the fo...
Questions

Spanish, 28.06.2019 07:30




Mathematics, 28.06.2019 07:30


Biology, 28.06.2019 07:30

History, 28.06.2019 07:30

Mathematics, 28.06.2019 07:30




History, 28.06.2019 07:30

Physics, 28.06.2019 07:30

Social Studies, 28.06.2019 07:30



Biology, 28.06.2019 07:30

Mathematics, 28.06.2019 07:30



