
Chemistry, 08.12.2020 05:50 bbyniah123
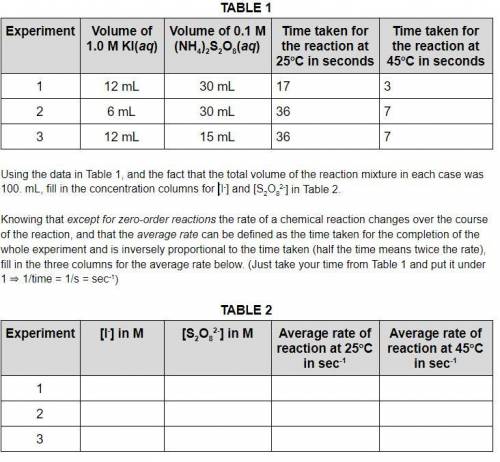
Using the data in Table 1, and the fact that the total volume of the reaction mixture in each case was 100. mL, fill in the concentration columns for [I-] and [S2O82-] in Table 2. Knowing that except for zero-order reactions the rate of a chemical reaction changes over the course of the reaction, and that the average rate can be defined as the time taken for the completion of the whole experiment and is inversely proportional to the time taken (half the time means twice the rate), fill in the three columns for the average rate below. (Just take your time from Table 1 and put it under 1 ⇒ 1/time = 1/s = sec-1)

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 07:30
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1

Chemistry, 22.06.2019 08:30
Which metal exist in liquid state and can be cut with knife ?
Answers: 2

You know the right answer?
Using the data in Table 1, and the fact that the total volume of the reaction mixture in each case w...
Questions


History, 17.04.2020 02:06

Social Studies, 17.04.2020 02:06


Arts, 17.04.2020 02:06

History, 17.04.2020 02:06


Computers and Technology, 17.04.2020 02:06



Arts, 17.04.2020 02:06




Mathematics, 17.04.2020 02:06

Mathematics, 17.04.2020 02:06






