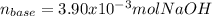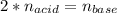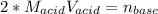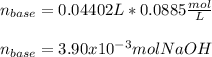Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 23.06.2019 03:00
A0.100-kilogram apple hangs in a tree 1.50 meter above the ground. ignore frictional effects, the total mechanical energy of the apples is
Answers: 1

Chemistry, 23.06.2019 06:10
How can liquids be seperated by density a the liquids are absorbed onto a paper b the liquids are turned into seperate vapors c the liquids are collected as they evaporate d the liquids are allowed to seperate into layers
Answers: 1

Chemistry, 23.06.2019 14:00
If you fill your car tire to a pressure of 32 psi (pounds per square inch) on a hot summer day when the temperature is 35°c (95°f), what is the pressure (in psi) on a cold winter day when the temperature is -15°c (5°f)? assume no gas leaks out between measurements and the volume of the tire does not change.
Answers: 1
You know the right answer?
g How many moles of NaOH are present in a sample if it is titrated to its equivalence point with 44....
Questions

German, 26.07.2019 19:30


Mathematics, 26.07.2019 19:30





Mathematics, 26.07.2019 19:30


History, 26.07.2019 19:30

English, 26.07.2019 19:30




Mathematics, 26.07.2019 19:30




English, 26.07.2019 19:30