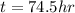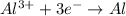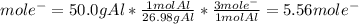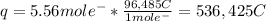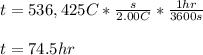Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 12:00
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal.b. he is determining chemical properties that are sufficient to identify the metal.c. he is determining physical properties that are insufficient to identify the metal.d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1
You know the right answer?
How long (in hours) will a current of 2.00 A need to be applied to produce 50.0 grams of aluminum me...
Questions


Geography, 20.11.2020 07:10

Mathematics, 20.11.2020 07:10

Biology, 20.11.2020 07:10

Mathematics, 20.11.2020 07:10

Mathematics, 20.11.2020 07:10




History, 20.11.2020 07:10





History, 20.11.2020 07:10


Business, 20.11.2020 07:10



Health, 20.11.2020 07:10