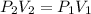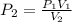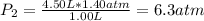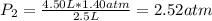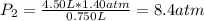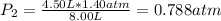Chemistry, 08.12.2020 16:50 itssergioa
The air in a 4.50 L tank has a pressure of 1.40 atm . What is the final pressure, in atmospheres, when the air is placed in tanks that have the following volumes, if there is no change in temperature and amount of gas?
a. 1.00 L
b. 2500. mL
c. 750. mL
d. 8.00 L

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:30
98 ! and brainliest plz ! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 23.06.2019 01:30
Concentrations expressed as a percent by mass are useful when the solute is a a. liquid b. gas c. solid
Answers: 1

Chemistry, 23.06.2019 02:00
Calculate the molarity of each aqueous solution: a. 78.0 ml of 0.240 m naoh diluted to 0.250 l with water b. 38.5 ml of 1.2 m hno3 diluted to 0.130 l with water
Answers: 1

Chemistry, 23.06.2019 03:20
High-pressure liquid chromatography (hplc) is a method used in chemistry and biochemistry to purify chemical substances. the pressures used in this procedure range from around 500 kilopascals (500,000 pa) to about 60,000 kpa (60,000,000 pa). it is often convenient to know the pressure in torr. if an hplc procedure is running at a pressure of 1.03×108 pa , what is its running pressure in torr?
Answers: 3
You know the right answer?
The air in a 4.50 L tank has a pressure of 1.40 atm . What is the final pressure, in atmospheres, wh...
Questions

Mathematics, 15.10.2019 10:30

Mathematics, 15.10.2019 10:30




Spanish, 15.10.2019 10:30




Mathematics, 15.10.2019 10:30

Mathematics, 15.10.2019 10:30


Computers and Technology, 15.10.2019 10:30

Biology, 15.10.2019 10:30


English, 15.10.2019 10:30


Mathematics, 15.10.2019 10:30

French, 15.10.2019 10:30