
Chemistry, 08.12.2020 16:50 luvberries2276
200.0 mL of 3.85 M HCl is added to 100.0 mL of 4.6 M barium hydroxide. The reaction goes to completion. What is the concentration (in mol/L) of excess H+ or OH- that remains in solution?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:00
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3
You know the right answer?
200.0 mL of 3.85 M HCl is added to 100.0 mL of 4.6 M barium hydroxide. The reaction goes to completi...
Questions

History, 19.07.2019 08:30

Health, 19.07.2019 08:30




History, 19.07.2019 08:30

Biology, 19.07.2019 08:30

SAT, 19.07.2019 08:30

Mathematics, 19.07.2019 08:30

Mathematics, 19.07.2019 08:30

Mathematics, 19.07.2019 08:30





Mathematics, 19.07.2019 08:30

French, 19.07.2019 08:30

Mathematics, 19.07.2019 08:30

Mathematics, 19.07.2019 08:30

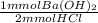 = 101.925 mmol Ba(OH)₂
= 101.925 mmol Ba(OH)₂

