Chemistry, 09.12.2020 01:30 frankgore8496
Calculate the pH for the following buffer solutions with steps.
1. 7.4862 grams of acetic acid and 10.4695 grams of sodium acetate are dissolved in enough deionized water to make 250.0 mL of solution.
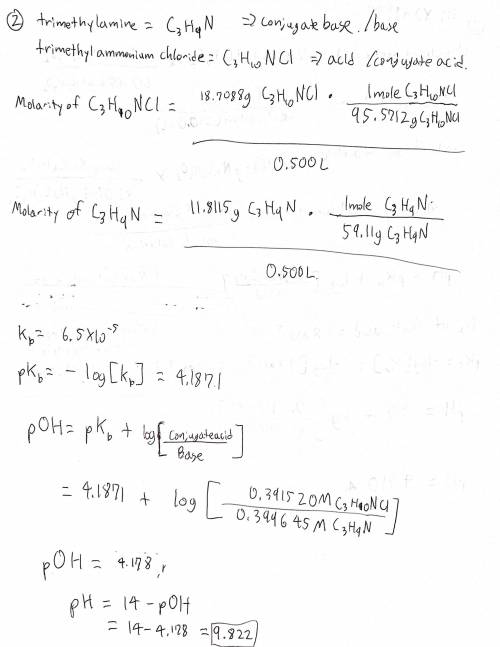
2. 11.8115 grams of trimethylamine and 18.7088 grams of trimethylammonium chloride are dissolved in enough deionized water to make 500.0 mL of solution.
Thank You:)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:00
The organism shown is a free-living one that is anchored to the bottom of ponds and streams during one stage of its life cycle what is the common name for the group to which this organism belong
Answers: 3

Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3

Chemistry, 22.06.2019 21:30
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2

Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1
You know the right answer?
Calculate the pH for the following buffer solutions with steps.
1. 7.4862 grams of acetic acid and...
Questions


Mathematics, 19.02.2021 02:50



Computers and Technology, 19.02.2021 02:50

Health, 19.02.2021 02:50

Mathematics, 19.02.2021 02:50

Computers and Technology, 19.02.2021 02:50


Social Studies, 19.02.2021 02:50





English, 19.02.2021 02:50


Mathematics, 19.02.2021 02:50