
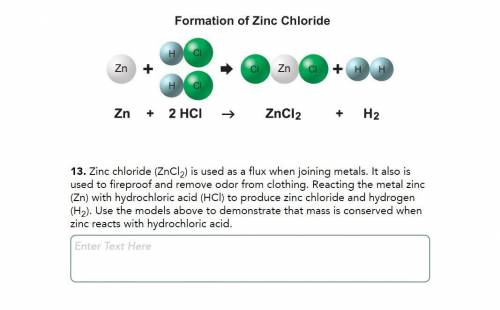
Zinc chloride (ZnCl2) is used as a flux when joining metals. It also is used to fireproof and remove odor from clothing. Reacting the metal zinc (Zn) with hydrochloric acid (HCl) to produce zinc chloride and hydrogen (H2). Use the models above to demonstrate that mass is conserved when zinc reacts with hydrochloric acid.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3

Chemistry, 22.06.2019 22:00
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3

Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3
You know the right answer?
Zinc chloride (ZnCl2) is used as a flux when joining metals. It also is used to fireproof and remove...
Questions






Computers and Technology, 30.11.2020 22:00

English, 30.11.2020 22:00


French, 30.11.2020 22:00



Mathematics, 30.11.2020 22:00



Mathematics, 30.11.2020 22:00

Mathematics, 30.11.2020 22:00


History, 30.11.2020 22:00

Mathematics, 30.11.2020 22:00



