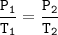Chemistry, 10.12.2020 01:00 chinyere614
The gases in a hairspray can are at a temperature of 27 C and a pressure of 30 psi. if the gases can reach a pressure of 90 psi, the can will explode. To what Temperature must the gases be raised in order for the can to explode? You can assume volume remains constant please help!!!

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 07:00
This image is an example of a(n) a) atom. b) compound. c) mixture. d) molecule.
Answers: 1

Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

You know the right answer?
The gases in a hairspray can are at a temperature of 27 C and a pressure of 30 psi. if the gases can...
Questions

English, 23.04.2020 21:35

Biology, 23.04.2020 21:35



Mathematics, 23.04.2020 21:35



Mathematics, 23.04.2020 21:35




Physics, 23.04.2020 21:35

English, 23.04.2020 21:35





Mathematics, 23.04.2020 21:36

Health, 23.04.2020 21:36