
Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 13:00
These questions are based on the attached photo. the experiment is about burning magnesium metal with oxygen. 1. write the balanced chemical equation for the reaction you are performing. 2. calculate the mass of magnesium metal used in each trial. o trial 1: o trial 2: 3. calculate the actual yield of magnesium oxide for each trial. o trial 1: o trial 2: 4. magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. o trial 1: o trial 2: 5. determine the percent yield of mgo for your experiment for each trial. o trial 1: o trial 2: 6. determine the average percent yield of mgo for the two trials. your company currently uses a process with a similar cost of materials that has an average percent yield of 91 percent. if the average percent yield of this process is higher than that, this could save the company money. what is your recommendation to the company? support your recommendation using your data, calculations, and understanding of stoichiometry gathered from this lab.
Answers: 1


You know the right answer?
Convert 4.92L of hydrogen at STP to moles...
Questions

Mathematics, 28.04.2021 20:00

Mathematics, 28.04.2021 20:00


Mathematics, 28.04.2021 20:00

Mathematics, 28.04.2021 20:00





Spanish, 28.04.2021 20:00


Mathematics, 28.04.2021 20:00

History, 28.04.2021 20:00

Mathematics, 28.04.2021 20:00






Biology, 28.04.2021 20:00

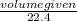
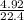 = 0.22moles
= 0.22moles

