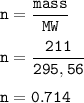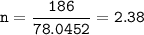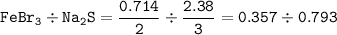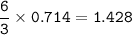A sample of 211 g of iron (III) bromide is reacted with
186 g of sodium sulfide to produce iron (III) sulfide
and sodium bromide. Using the balanced equation
below, predict which is the limiting reactant and the
maximum amount in moles of sodium bromide that
can be produced.
2FeBr3 + 3Na2S → Fe2S3 + 6NaBr

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:30
The boiling point of liquids is very high what does it indicate
Answers: 1

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

Chemistry, 22.06.2019 14:30
Aroom with dimensions 7.00m×8.00m×2.50m is to be filled with pure oxygen at 22.0∘c and 1.00 atm. the molar mass of oxygen is 32.0 g/mol. how many moles noxygen of oxygen are required to fill the room? what is the mass moxygen of this oxygen?
Answers: 1
You know the right answer?
A sample of 211 g of iron (III) bromide is reacted with
186 g of sodium sulfide to produce iron (II...
Questions


Mathematics, 22.02.2021 20:20

Computers and Technology, 22.02.2021 20:20

History, 22.02.2021 20:20


Mathematics, 22.02.2021 20:20

English, 22.02.2021 20:20

Biology, 22.02.2021 20:20



English, 22.02.2021 20:20

Biology, 22.02.2021 20:20




History, 22.02.2021 20:20


History, 22.02.2021 20:20