
Chemistry, 10.12.2020 01:40 angelmosby9
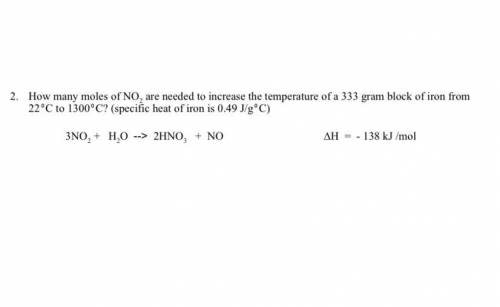
How many moles of NO2 are needed to increase the temperature of a 333 gram block of iron from 220C to 13000C? (specific heat of iron is 0.49 J/g0C) 3NO2 + H2O --> 2HNO3 + NO ∆H = - 138 kJ /mol

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:00
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1


Chemistry, 22.06.2019 10:00
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1
You know the right answer?
How many moles of NO2 are needed to increase the temperature of a 333 gram block of iron from 220C t...
Questions


Spanish, 31.07.2019 20:00

Mathematics, 31.07.2019 20:00

Mathematics, 31.07.2019 20:00



Spanish, 31.07.2019 20:00

History, 31.07.2019 20:00

Social Studies, 31.07.2019 20:00









English, 31.07.2019 20:00

Physics, 31.07.2019 20:00



