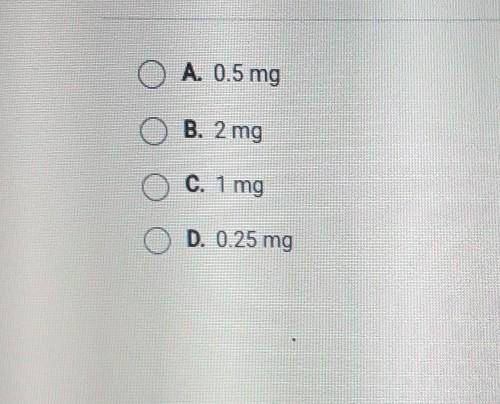
How much Cu-64 (half-life about 12 hours) would remain from a 2 mg sample after 12 hours?
...

Chemistry, 10.12.2020 03:20 vivian2020
How much Cu-64 (half-life about 12 hours) would remain from a 2 mg sample after 12 hours?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3

Chemistry, 23.06.2019 03:00
Analyze the reaction to determine whether the reaction is exothermic or endothermic. explain your reasoning.
Answers: 1

Chemistry, 23.06.2019 04:20
The reaction below shows a system in equilibrium. how would a decrease in temperature affect this reaction? a. the rate of formation of the gases would increase. b. the equilibrium of the reaction would shift to the left. c. the equilibrium would shift to cause the gases to sublime into solids. d. the chemicals on the left would quickly form the chemical on the right.
Answers: 1

Chemistry, 23.06.2019 06:30
The polarity of an oxygen-hydrogen bond is higher than the polarity of a nitrogen-hydrogen bond, allowing amines to be more soluble than alcohols.
Answers: 3
You know the right answer?
Questions

Chemistry, 09.01.2021 01:00


English, 09.01.2021 01:00


Computers and Technology, 09.01.2021 01:00

Mathematics, 09.01.2021 01:00

Chemistry, 09.01.2021 01:00


Chemistry, 09.01.2021 01:00


Mathematics, 09.01.2021 01:00

Mathematics, 09.01.2021 01:00


Mathematics, 09.01.2021 01:00

Mathematics, 09.01.2021 01:00


Mathematics, 09.01.2021 01:00





