
Chemistry, 10.12.2020 04:00 lizzyhearts
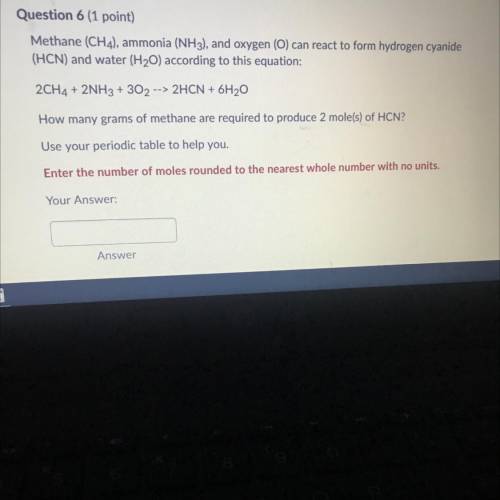
Methane (CH 4 ) , ammonia (NH 3 ) , and oxygen () can reaci to form hydrogen cyanide (HCN) and water (H 2 O) according to this equation 2CH 4 +2NH 3 +3O 2 2HCN+6H 2 O How many grams of methane are required to produce 2 moles ? Use your periodic table to help you. Please show work

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 22.06.2019 15:30
How does a large body of water, such as the ocean, influence climate?
Answers: 1

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1
You know the right answer?
Methane (CH 4 ) , ammonia (NH 3 ) , and oxygen () can reaci to form hydrogen cyanide (HCN) and water...
Questions

Mathematics, 29.11.2021 23:50






Mathematics, 29.11.2021 23:50

Mathematics, 29.11.2021 23:50

Mathematics, 29.11.2021 23:50




SAT, 29.11.2021 23:50

Mathematics, 29.11.2021 23:50


Mathematics, 29.11.2021 23:50


Mathematics, 29.11.2021 23:50




