
Chemistry, 10.12.2020 06:20 jonathon3957
URGENT PLEASE HELP!
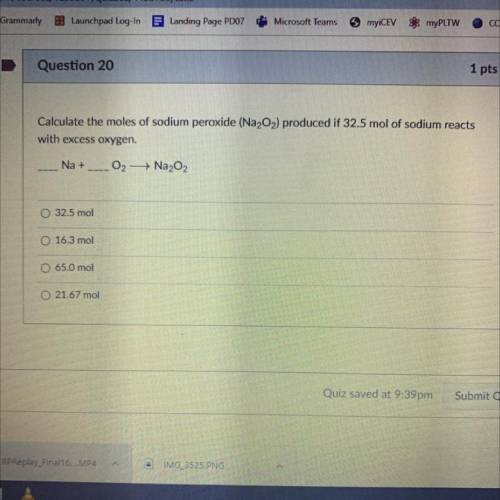
Calculate the moles of Sodium Peroxide (Na2O2) produced if 32.5 mol of sodium reacts with excess oxygen.
__Na+__O2—>Na2O2
A. 32.5 mol
B. 16.3 mol
C. 65.0 mol
D. 21.67 mol
and why is it one of the answer above?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? )
Answers: 3

Chemistry, 21.06.2019 22:30
Explain why scientists use shared characteristics to make cladograms.
Answers: 1

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3
You know the right answer?
URGENT PLEASE HELP!
Calculate the moles of Sodium Peroxide (Na2O2) produced if 32.5 mol of sodium r...
Questions

Mathematics, 15.04.2021 14:00


Mathematics, 15.04.2021 14:00

Business, 15.04.2021 14:00


Mathematics, 15.04.2021 14:00




Physics, 15.04.2021 14:00

Mathematics, 15.04.2021 14:00


Mathematics, 15.04.2021 14:00



English, 15.04.2021 14:00

Physics, 15.04.2021 14:00



Chemistry, 15.04.2021 14:00



