

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:40
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1

Chemistry, 22.06.2019 08:20
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3

Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Chemistry, 22.06.2019 15:20
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1
You know the right answer?
Calculate the maximum volume in mL of 0.20 M HCl that a tablet containing 338 mg Al(OH)3 and 489 mg...
Questions

History, 24.09.2019 10:30



Mathematics, 24.09.2019 10:30

Biology, 24.09.2019 10:30

Mathematics, 24.09.2019 10:30


Mathematics, 24.09.2019 10:30

Health, 24.09.2019 10:30

Mathematics, 24.09.2019 10:30

Business, 24.09.2019 10:30




Biology, 24.09.2019 10:30


History, 24.09.2019 10:30

Mathematics, 24.09.2019 10:30


Social Studies, 24.09.2019 10:30

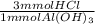 = 13 mmol HCl
= 13 mmol HCl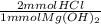 = 16.77 mmol HCl
= 16.77 mmol HCl

