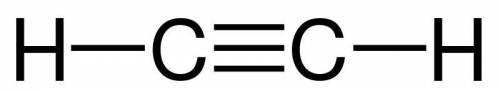Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:10
Identify one disadvantage to each of the following models of electron configuration: dot structures arrow and line diagrams written electron configurations type in your answer below.
Answers: 1

Chemistry, 21.06.2019 23:40
If the atomic mass of an atom is 34 and the atom contains 13 protons, how many neutrons does the atom contain?
Answers: 2

Chemistry, 22.06.2019 00:00
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1
You know the right answer?
Draw the structural formula for acetylene, C2H2, and state the type of bonds in an acetylene molecul...
Questions


Mathematics, 22.05.2020 02:07


Mathematics, 22.05.2020 02:07

Mathematics, 22.05.2020 02:07


Law, 22.05.2020 02:07




History, 22.05.2020 02:07


Mathematics, 22.05.2020 02:07

Biology, 22.05.2020 02:07

Mathematics, 22.05.2020 02:07



Medicine, 22.05.2020 02:07

Mathematics, 22.05.2020 02:07

Spanish, 22.05.2020 02:07