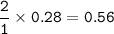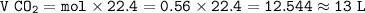Chemistry, 10.12.2020 19:50 anthonybowie99
HELP FOR FINAL
In an industrial process ethanol C2H6o burns with Oz to produce heat.
C2H5OH + 3 o2 - 2 CO2 + 3 H20 + 8842 Joules
How many liters of CO2 are obtained from burning 0.28 moles of ethanol at STP (standard temperature and pressure? Express your result with 2 significant figures.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:30
150.0 grams of asf3 were reacted with 180.0 g of ccl4 to produce ascl3 and ccl2f2. if the actual yield of ccl2f2 was 127 g, what is the percent yield?
Answers: 2

Chemistry, 21.06.2019 23:00
Which statement describes covalent bases? they have hydroxide ions. they produce hydrogen ions. they are often amines. they are named the same as ionic compounds.
Answers: 3

Chemistry, 22.06.2019 00:40
Which is a difference between molecular compounds and ionic compounds? select the correct answer below: question 5 options: molecular compounds typically form between a metal and a nonmetal, while ionic compounds typically form between nonmetals. molecular compounds result from the transfer of electrons between atoms to form ions, while ionic compounds result from the sharing of electrons between neutral atoms. molecular compounds are formed of discrete, neutral molecules, while ionic compounds are formed of large repeating arrays of opposite charges. molecular compounds have high melting points and high boiling points, while ionic
Answers: 3

You know the right answer?
HELP FOR FINAL
In an industrial process ethanol C2H6o burns with Oz to produce heat.
C2H5OH +...
C2H5OH +...
Questions





Mathematics, 12.03.2020 20:25




Chemistry, 12.03.2020 20:25







Health, 12.03.2020 20:25

Mathematics, 12.03.2020 20:25