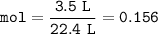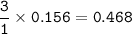Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1

Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1
You know the right answer?
When nitrogen gas (N2) reacts with hydrogen gas(H) ammonia gas (NH3) is formed. How many grams of hy...
Questions

Mathematics, 10.09.2021 20:30

Mathematics, 10.09.2021 20:30


Health, 10.09.2021 20:30





Mathematics, 10.09.2021 20:30



Mathematics, 10.09.2021 20:30

Mathematics, 10.09.2021 20:30


Mathematics, 10.09.2021 20:30