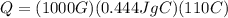Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1

Chemistry, 23.06.2019 02:00
Calculate the molarity of each aqueous solution: a. 78.0 ml of 0.240 m naoh diluted to 0.250 l with water b. 38.5 ml of 1.2 m hno3 diluted to 0.130 l with water
Answers: 1
You know the right answer?
A 1000-g iron frying pan is placed on a stove. The pan increases from 20°C to 130°C. If the specific...
Questions

Mathematics, 05.12.2019 05:31

Mathematics, 05.12.2019 05:31




Mathematics, 05.12.2019 05:31




Mathematics, 05.12.2019 05:31

Biology, 05.12.2019 05:31

Mathematics, 05.12.2019 05:31

Mathematics, 05.12.2019 05:31



Mathematics, 05.12.2019 05:31

Mathematics, 05.12.2019 05:31



Mathematics, 05.12.2019 05:31