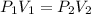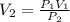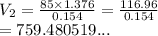Chemistry, 11.12.2020 01:00 jordaaan101
When 85 L of gas at 1.376 atm has its pressure changed to 0.154 atm, what is the new volume?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 22:00
11) burning your hand when accidentally touching a hot plate is an example of which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 2

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1

Chemistry, 23.06.2019 06:50
What is the volume of 3.2 moles of chlorine gas (cl2) at 295 k and 1.1 atm?
Answers: 1
You know the right answer?
When 85 L of gas at 1.376 atm has its pressure changed to 0.154 atm, what is the new volume?...
Questions

Chemistry, 20.09.2020 20:01



Mathematics, 20.09.2020 20:01





Mathematics, 20.09.2020 20:01

Physics, 20.09.2020 20:01

English, 20.09.2020 20:01

Biology, 20.09.2020 20:01

English, 20.09.2020 20:01

Mathematics, 20.09.2020 20:01

History, 20.09.2020 20:01


Mathematics, 20.09.2020 20:01


Business, 20.09.2020 20:01