
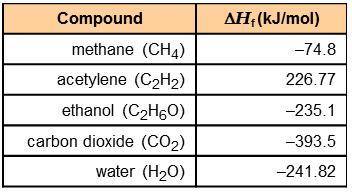
Using the information in the table to the right, calculate the enthalpy of combustion of each of the following substances:
acetylene:
ethanol:
The combustion of 0.25 mol of an unknown organic compound results in the release of 320 kJ of energy. Which of the compounds in the table could be the unknown compound?
ANSWERS:
1.
acetylene: -1,256 kJ/mol
ethanol: -1,277 kJ/mol
2.
ethanol
I already know the answers (they're right there ^) i just need to know HOW you find the enthalpy combustion of acetylene and ethanol.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1

Chemistry, 23.06.2019 01:20
How can parts of a solution be separated by chromatography?
Answers: 1

Chemistry, 23.06.2019 02:00
What is the difference between a substance "getting wet" and "being dissolved" in a liquid at the particulate level?
Answers: 3
You know the right answer?
Using the information in the table to the right, calculate the enthalpy of combustion of each of the...
Questions

Mathematics, 29.11.2019 03:31

Mathematics, 29.11.2019 03:31





Physics, 29.11.2019 03:31

History, 29.11.2019 03:31



Mathematics, 29.11.2019 03:31



French, 29.11.2019 03:31



Mathematics, 29.11.2019 03:31


Biology, 29.11.2019 03:31

Mathematics, 29.11.2019 03:31



