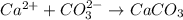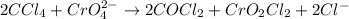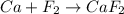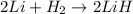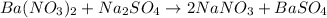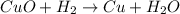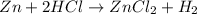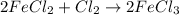Chemistry, 11.10.2019 04:30 julianbeaver76
Classify these reactions according to the type discussed in the chapter.
b) ca2+ + co32- -> caco3
d) 2ccl4 + cro42- -> 2cocl2+ cro2cl2+2cl-
e) ca+ f2-> caf2
f) 2li+h2-> 2lih
g) ba(no3)2+na2so4-> 2nano3+baso4
h) cuo+h2-> cu+h20
i) zn+2hcl-> zncl2+h2
j) 2fecl2+cl2-> 2fecl3

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which type of reaction always has an element and a compound as reactants
Answers: 1

Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2


Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3
You know the right answer?
Classify these reactions according to the type discussed in the chapter.
b) ca2+ + co32...
b) ca2+ + co32...
Questions


Mathematics, 17.01.2021 09:00

Geography, 17.01.2021 09:00

Biology, 17.01.2021 09:00

Mathematics, 17.01.2021 09:00


Mathematics, 17.01.2021 09:00

English, 17.01.2021 09:00

Mathematics, 17.01.2021 09:00







Mathematics, 17.01.2021 09:00


Social Studies, 17.01.2021 09:00


English, 17.01.2021 09:00