
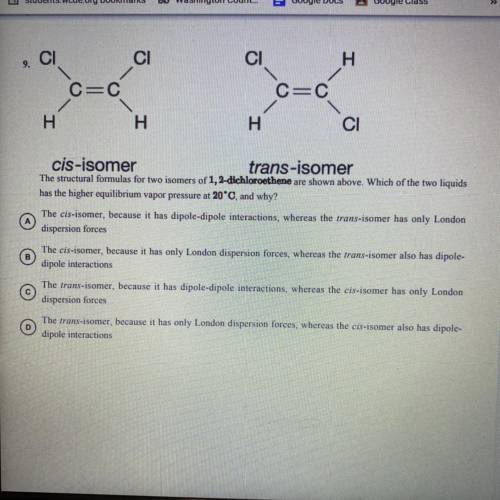
The structural formulas for two isomers of 1, 2-dichloroethene are shown above. Which of the two liquids has the higher equilibrium vapor pressure at 20 degrees Celsius and why?
A) the cis-isomer, because it has dipole-dipole interactions, whereas the trans-isomer has only 1 London dispersion forces.
B) the cis-isomer, because it has only London dispersion forces, whereas the trans-isomer also has dipole-dipole interactions
C) the trans-isomer, because it has dipole-dipole interactions, whereas the cis-isomer has only 1 London dispersion forces
D) the trans-isomer, because it has only 1 London dispersion forces, whereas the cos-isomer also has dipole-dipole interactions

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2


Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3
You know the right answer?
The structural formulas for two isomers of 1, 2-dichloroethene are shown above. Which of the two liq...
Questions

Mathematics, 21.04.2020 16:58

Mathematics, 21.04.2020 16:58

Mathematics, 21.04.2020 16:58



















