
Chemistry, 12.12.2020 15:50 nathancikra
5.00 ml of an H2SO4 solution with unknown molarity was diluted with 25.00 ml. This was tirated with 15.00ml of .1000 M NaOH to reach the equivalence point. What is the molarity of the original unknown H2SO4 solution? Also, what would the balanced chemical equation for this reaction be?

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2

Chemistry, 22.06.2019 15:30
Light waves can move through , but they travel fastest when they move through a(n) .
Answers: 1

You know the right answer?
5.00 ml of an H2SO4 solution with unknown molarity was diluted with 25.00 ml. This was tirated with...
Questions


Mathematics, 30.09.2019 07:50

History, 30.09.2019 07:50

Mathematics, 30.09.2019 07:50


History, 30.09.2019 07:50


Mathematics, 30.09.2019 07:50






Chemistry, 30.09.2019 07:50




Mathematics, 30.09.2019 07:50

Mathematics, 30.09.2019 07:50

History, 30.09.2019 07:50

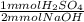 = 0.75 mmol H₂SO₄
= 0.75 mmol H₂SO₄

