
Chemistry, 21.09.2019 01:40 dbenjamintheflash5
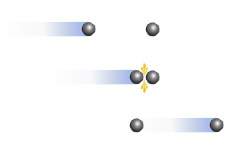
Consider the diagram. which postulate of the kinetic-molecular theory best describes the event in the diagram?
a. most of the volume of a gas is empty space.
b. all collisions between particles are perfectly elastic.
c. there is no force of attraction or repulsion between gas particles.
d. the average kinetic energy of particles depends only on temperature.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Consider the nuclear equation below. 239 > x + 4 he 94 2 what is x? 1.235 cm 96 2.243 u 92 3.235 u 92 4.243 cm 96
Answers: 2

Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3

Chemistry, 23.06.2019 10:00
Abike ride event is 30 miles. a first aid tent is put at the 3/4 mark of the course. how many miles from the starting point is the first aid tent?
Answers: 1

Chemistry, 23.06.2019 10:30
How much mass would a mole of hydrogen molecules contain? recall that hydrogen is diatomic. g/mol
Answers: 3
You know the right answer?
Consider the diagram. which postulate of the kinetic-molecular theory best describes the event in th...
Questions





Physics, 21.09.2019 03:10



Mathematics, 21.09.2019 03:10


Biology, 21.09.2019 03:10


English, 21.09.2019 03:10



Social Studies, 21.09.2019 03:10



English, 21.09.2019 03:10

Chemistry, 21.09.2019 03:10

Health, 21.09.2019 03:10



