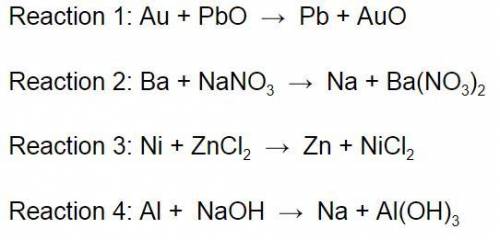
Using the Activity Series of Metals, which of the reactions below would most likely occur? *
...

Chemistry, 12.12.2020 16:00 austinpedigo
Using the Activity Series of Metals, which of the reactions below would most likely occur? *

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 15:40
What is the total reduction potential of a cell in which potassium (k) is reduced and copper (cu) is oxidized? a. 2.59 v b. 3.27 v c. -3.27 v d.-2.59 v
Answers: 1


Chemistry, 22.06.2019 08:30
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1

Chemistry, 22.06.2019 10:00
Drug abuse will not lead to physical and psychological dependence. true or false ?
Answers: 2
You know the right answer?
Questions


French, 07.09.2021 01:50

Mathematics, 07.09.2021 01:50

Mathematics, 07.09.2021 01:50

Chemistry, 07.09.2021 01:50

Mathematics, 07.09.2021 01:50


Mathematics, 07.09.2021 01:50

Mathematics, 07.09.2021 01:50

Mathematics, 07.09.2021 01:50




Spanish, 07.09.2021 01:50

Social Studies, 07.09.2021 01:50








