
Chemistry, 12.12.2020 16:30 smartgirl2092
PLZ HELP
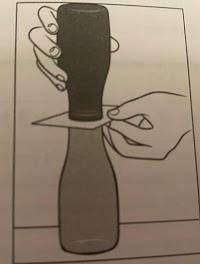
A group of students simulates colliding air masses. Using an index card for separation, a jar of warm tap water with red food coloring is placed over a jar of cold water with yellow food coloring. The index card that separates the two bodies of water is removed. Students observe how the two fronts travel in relation to one another.
A.) Air masses are not distinguisable by color.
B.) Air masses are neither hot nor cold in temperature
C.) Air masses do not collide in real life
D.) Air masses cannot be modeled

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3


Chemistry, 22.06.2019 18:30
How many moles of lead are in 1.50 x 10^12 atoms of lead? could you explain the answer as well and not just give it to me i am refreshing for finals and i need to know how to do it
Answers: 3
You know the right answer?
PLZ HELP
A group of students simulates colliding air masses. Using an index card for separation, a...
Questions

Social Studies, 07.01.2020 11:31

Mathematics, 07.01.2020 11:31


Social Studies, 07.01.2020 11:31

Mathematics, 07.01.2020 11:31

English, 07.01.2020 11:31

Mathematics, 07.01.2020 11:31

Mathematics, 07.01.2020 11:31

History, 07.01.2020 11:31

Mathematics, 07.01.2020 11:31






Mathematics, 07.01.2020 11:31



Social Studies, 07.01.2020 11:31



