
Chemistry, 12.12.2020 16:30 animaljamissofab
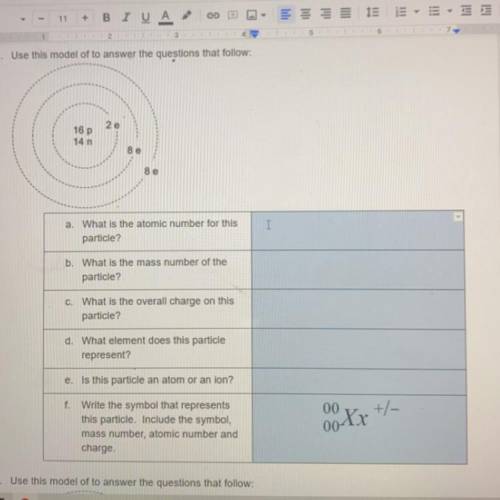
A. What is the atomic number for this particle
B. What is the mass number of the particle
C. what is the overall charge on this particle
D. What element does this particle represent
E. Is this particle an atom or an ion
F. Write the symbol that represents this particle. Include the symbol, mass number, atomic number and charge

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:20
Which of the following statements is false regarding aromaticity? a. the compound must be cyclic b. the compound must be fully conjugated c. the compound must be planar d.the number of electrons in the pi system must satisfy the hückel 4n+2 rule e. the compound must have a neutral charge
Answers: 2

Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3

Chemistry, 22.06.2019 20:30
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2

Chemistry, 22.06.2019 21:00
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1
You know the right answer?
A. What is the atomic number for this particle
B. What is the mass number of the particle
Questions

Spanish, 09.01.2021 09:00

Biology, 09.01.2021 09:00


Business, 09.01.2021 09:00


Spanish, 09.01.2021 09:00

Mathematics, 09.01.2021 09:00

Mathematics, 09.01.2021 09:00

Mathematics, 09.01.2021 09:00


Physics, 09.01.2021 09:00



Social Studies, 09.01.2021 09:00

Physics, 09.01.2021 09:00


Mathematics, 09.01.2021 09:10

English, 09.01.2021 09:10

Mathematics, 09.01.2021 09:10

Biology, 09.01.2021 09:10



