
Chemistry, 12.12.2020 16:40 Hosanna130
Consider the following intermediate chemical equations.
What is the enthalpy of the overall chemical reaction?
-999 kJ
-250 kJ
250 kJ
999 kJ
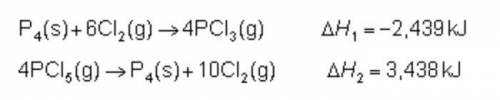
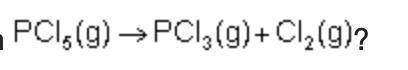

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:10
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3
You know the right answer?
Consider the following intermediate chemical equations.
What is the enthalpy of the overall chemica...
Questions



Mathematics, 02.02.2022 16:50



Business, 02.02.2022 16:50

History, 02.02.2022 16:50

Computers and Technology, 02.02.2022 16:50


SAT, 02.02.2022 16:50

Mathematics, 02.02.2022 16:50



Mathematics, 02.02.2022 16:50


Geography, 02.02.2022 16:50

SAT, 02.02.2022 17:00






