
Chemistry, 12.12.2020 16:50 yarbor800592
Hydrazine reacts with oxygen according to the following equation: N2H4(g) +O2(g) → N2(g) + 2 H2O(l) How many L of N2, measured at 34.9 °C and 755.08 torr, will be produced at the same time that 914.894 g of H2O is produced?

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 05:30
The climate of the continental united states is generally 1. tropical 2. temperate 3. arctic 4. highland
Answers: 1

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 22:30
What relationship exists between an enzyme and a catalyst?
Answers: 1
You know the right answer?
Hydrazine reacts with oxygen according to the following equation: N2H4(g) +O2(g) → N2(g) + 2 H2O(l)...
Questions

Health, 12.11.2020 20:40


Mathematics, 12.11.2020 20:40



Mathematics, 12.11.2020 20:40

English, 12.11.2020 20:40

Mathematics, 12.11.2020 20:40


Mathematics, 12.11.2020 20:40


Mathematics, 12.11.2020 20:40

Chemistry, 12.11.2020 20:40




Engineering, 12.11.2020 20:40


Mathematics, 12.11.2020 20:40

Chemistry, 12.11.2020 20:40

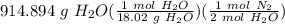 = 25.3955 mol N₂
= 25.3955 mol N₂

