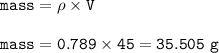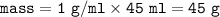Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3


Chemistry, 22.06.2019 22:30
Which of the following is true about the speed of light? it depends on the wavelength.
Answers: 3
You know the right answer?
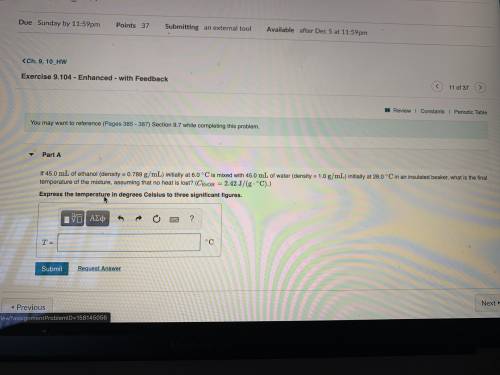
If 45.0 mL of ethanol (density =0.789g/mol) initially at 6.0°C mix with 45.0 mL of water (density =1...
Questions





History, 17.07.2019 00:40



Mathematics, 17.07.2019 00:40

English, 17.07.2019 00:40

History, 17.07.2019 00:40


Mathematics, 17.07.2019 00:40


Mathematics, 17.07.2019 00:40

Mathematics, 17.07.2019 00:40


Mathematics, 17.07.2019 00:40