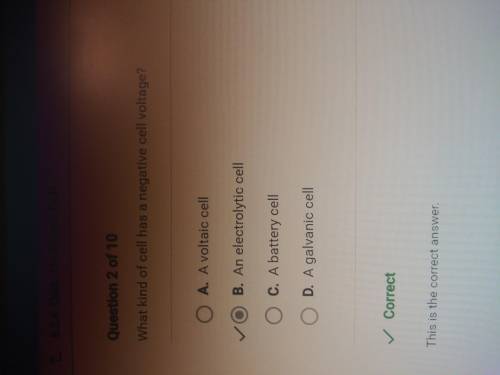
What kind of cell has a negative cell voltage?
A. A voltaic cell
B. A battery cell
C. A...


Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 22:00
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1

Chemistry, 22.06.2019 23:50
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3

Chemistry, 23.06.2019 00:20
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1

Chemistry, 23.06.2019 10:30
The element chlorine has two stable isotopes, chlorine-35 with a mass of 34.97 amu and chlorine-37 with a mass of 36.95 amu. from the atomic weight of cl = 35.45 one can conclude that:
Answers: 2
You know the right answer?
Questions







Mathematics, 23.01.2021 01:00


Mathematics, 23.01.2021 01:00

Social Studies, 23.01.2021 01:00

Mathematics, 23.01.2021 01:00

Mathematics, 23.01.2021 01:00



Mathematics, 23.01.2021 01:00

Geography, 23.01.2021 01:00

Geography, 23.01.2021 01:00

Mathematics, 23.01.2021 01:00

Mathematics, 23.01.2021 01:00

History, 23.01.2021 01:00