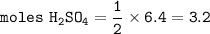Chemistry, 13.12.2020 23:50 muziqbox594
15. Upon balancing the equation below, how many moles of sulfuric acid are needed to react completely with 6.4 moles of lithium hydroxide?
LIOH + H2SO4 -> Li2SO4 + H20 (2 points)
12.8 mol H2SO4
6.4 mol H2SO4
O 3.2 mol H2SO4
O 2.1 mol H2SO4

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 02:30
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2

Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2
You know the right answer?
15. Upon balancing the equation below, how many moles of sulfuric acid are needed to react completel...
Questions


History, 21.09.2019 02:00

Social Studies, 21.09.2019 02:00



Social Studies, 21.09.2019 02:00

Physics, 21.09.2019 02:00

Mathematics, 21.09.2019 02:00


History, 21.09.2019 02:00







Computers and Technology, 21.09.2019 02:00


Mathematics, 21.09.2019 02:00