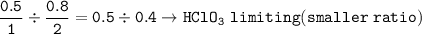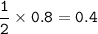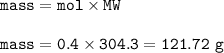You are logged in as Lara Khader
y (1)
Question 2
Not yet answered
Marked out of...

You are logged in as Lara Khader
y (1)
Question 2
Not yet answered
Marked out of 1.0
The mass of Ba(CIO), formed when 0.50 mole of Ba(OH), is treated with 0.80 mol of HCIO, according to the reaction below is
Ba(OH), + 2HCIO, > Ba(CIO3)2 + 2H,0
Ba: 137.3 g/mol
O: 16 g/mol
H: 1 g/mol
Cl: 35.45 g/mol
Flag question
16
Select one:
304 2 g
243.4 g
121.79
380.7 g
None of the above
Next page

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:00
If 1.63 times 10 negative 4 of helium dissolves in 100.0g of water, what is the concentration in parts per million
Answers: 3

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3
You know the right answer?
Questions

Mathematics, 05.05.2020 12:45

Mathematics, 05.05.2020 12:45

Biology, 05.05.2020 12:45



Biology, 05.05.2020 12:45

Mathematics, 05.05.2020 12:45

Mathematics, 05.05.2020 12:45


English, 05.05.2020 12:46


Advanced Placement (AP), 05.05.2020 12:46


Mathematics, 05.05.2020 12:46

Biology, 05.05.2020 12:46

Mathematics, 05.05.2020 12:46


Mathematics, 05.05.2020 12:46

Chemistry, 05.05.2020 12:46

Mathematics, 05.05.2020 12:46