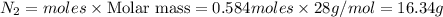What mass of Nz will be needed to produce 31.5 grams of N2O5?
4N2 + 502 --> 2N2O5
a) 158.3...

Chemistry, 14.12.2020 16:30 rustalex6045
What mass of Nz will be needed to produce 31.5 grams of N2O5?
4N2 + 502 --> 2N2O5
a) 158.3 grams
b) 38.64 grams
c) 4.96 grams
d) 16.34 grams

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 18:30
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

Chemistry, 23.06.2019 15:40
The poh of a solution is 6.0. which statement is correct? use poh=-log[oh and ph+poh= 14 o o o the ph of the solution is 20.0. the concentration of oh ions is 1.0 x 10-8 m- the concentration of oh ions is 1.0 x 106 m- the ph of the solution is 8.0.
Answers: 3

Chemistry, 23.06.2019 21:00
Examine these two msds from different manufacturers for sodium hydroxide (naoh). compare and contrast the following aspects: chemical names, chemical properties, order of components, health hazards, and proper disposal. click on each of the links to examine two msds reports from different sources. sodium hydroxide version 1 sodium hydroxide version 2
Answers: 1

Chemistry, 23.06.2019 21:30
Hno3 + s → h2so4 + no break down the equation shown into the skeletal half-reactions for oxidation and reduction. which of these pairs shows the two skeletal half-reactions with their correct assignments?
Answers: 3
You know the right answer?
Questions

Social Studies, 10.02.2021 23:20



Mathematics, 10.02.2021 23:20

History, 10.02.2021 23:20

Mathematics, 10.02.2021 23:20

Mathematics, 10.02.2021 23:20



Engineering, 10.02.2021 23:20

Mathematics, 10.02.2021 23:20

History, 10.02.2021 23:20



Mathematics, 10.02.2021 23:20



Social Studies, 10.02.2021 23:20

Mathematics, 10.02.2021 23:20

Chemistry, 10.02.2021 23:20

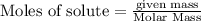
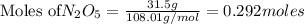
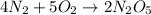
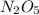 are produced by= 4 moles of
are produced by= 4 moles of 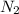
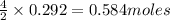 of
of