
Chemistry, 14.12.2020 18:20 research73
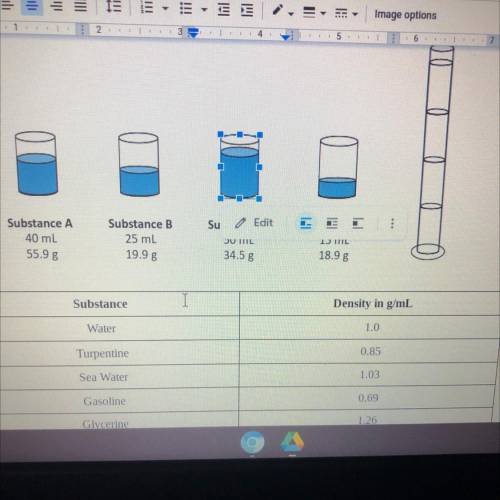
A chemistry class is given four beakers containing unknown liquids A, B, C, and shown below.
Address each of the following prompts in a one page essay below.
• Predict what would happen if liquids A, B,C, and D were added to the graduated cylinder
and use quantitative data as evidence to support your prediction.
• Describe the graduated cylinder with the names of each substance and the order in
which they would occur based on their densities.
• Finally, predict where a sphere with a mass of 2.5g and a volume of 2.33 cm would stop
in the column. Justify your reasoning with quantitative data.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
How many moles are in 250 grams of tungsten (w)? * 4.4x10^23 moles 4.2x10^23 moles 0.7 moles 1.4 moles
Answers: 3


Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

You know the right answer?
A chemistry class is given four beakers containing unknown liquids A, B, C, and shown below.
Addres...
Questions

Mathematics, 18.12.2020 19:00


Mathematics, 18.12.2020 19:00

History, 18.12.2020 19:00

Mathematics, 18.12.2020 19:00

Chemistry, 18.12.2020 19:00

Mathematics, 18.12.2020 19:00


Chemistry, 18.12.2020 19:00



Mathematics, 18.12.2020 19:00


Mathematics, 18.12.2020 19:00



Mathematics, 18.12.2020 19:00

Chemistry, 18.12.2020 19:00


Arts, 18.12.2020 19:00



