
Chemistry, 14.12.2020 20:30 clietmaster112
Gas law A gas has a volume of 350 mL at 4.5 ATM. If the volume changes to 400 mL, what is the new pressure? The temperature in the number of moles stay the same.
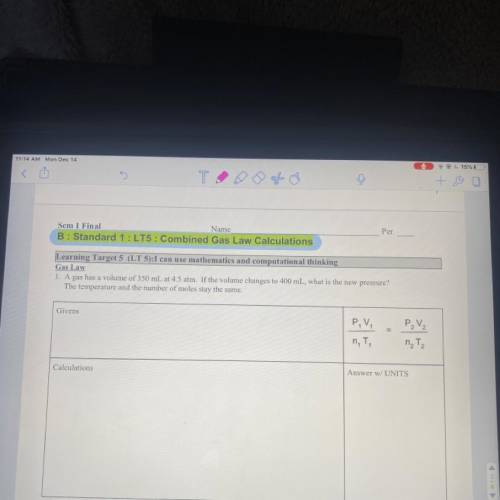

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:40
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1

Chemistry, 23.06.2019 00:30
What is bromine+calcium iodide--> calcium bromide +iodine balanced
Answers: 1

Chemistry, 23.06.2019 02:00
Anitrogen atom and an oxygen atom combine chemically to form nitric oxide. what is nitric oxide?
Answers: 1
You know the right answer?
Gas law
A gas has a volume of 350 mL at 4.5 ATM. If the volume changes to 400 mL, what is the new p...
Questions





Biology, 20.09.2019 19:30

History, 20.09.2019 19:30

Mathematics, 20.09.2019 19:30

Mathematics, 20.09.2019 19:30





Biology, 20.09.2019 19:30

English, 20.09.2019 19:30

Mathematics, 20.09.2019 19:30


History, 20.09.2019 19:30


Mathematics, 20.09.2019 19:30



