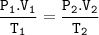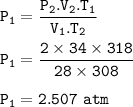2. A gas that has a volume of 28 liters, a ternperature of 45 °C, and an unknown
pressure has its volume increased to 34 iters and its temperature decreased to
35 °C. If I measure the pressure after the change to be 2.0 atm, what was the
original pressure of the gas?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1

Chemistry, 22.06.2019 05:30
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3
You know the right answer?
2. A gas that has a volume of 28 liters, a ternperature of 45 °C, and an unknown
pressure has its v...
Questions


Physics, 09.01.2022 14:00

English, 09.01.2022 14:00


Mathematics, 09.01.2022 14:00

Mathematics, 09.01.2022 14:00




Chemistry, 09.01.2022 14:00



History, 09.01.2022 14:00

Business, 09.01.2022 14:10


Arts, 09.01.2022 14:10

Mathematics, 09.01.2022 14:10