
Chemistry, 14.12.2020 22:50 ritasolomon85
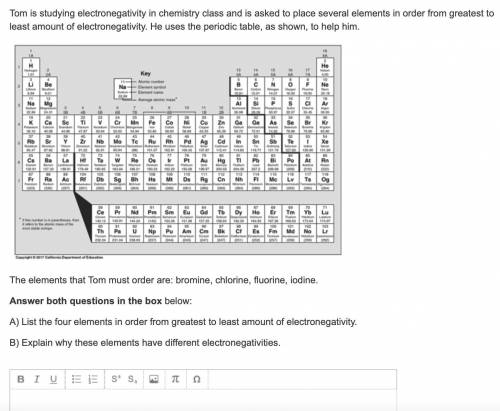
Tom is studying electronegativity in chemistry class and is asked to place several elements in order from greatest to least amount of electronegativity. He uses the periodic table, as shown, to help him.
The elements that Tom must order are: bromine, chlorine, fluorine, iodine.
Answer both questions in the box below:
A) List the four elements in order from greatest to least amount of electronegativity.
B) Explain why these elements have different electronegativities.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Which of the following compounds does not contain molecules? question 2 options: co2 h2 nacl h2o
Answers: 1

Chemistry, 21.06.2019 22:00
Fission of uranium-235 products energy and a. isotopes of smaller elements b. isotopes of larger elements c. lighter isotopes of uranium d. heavier isotopes of uranium
Answers: 3

Chemistry, 22.06.2019 07:20
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1
You know the right answer?
Tom is studying electronegativity in chemistry class and is asked to place several elements in order...
Questions

Mathematics, 06.02.2021 01:50


Mathematics, 06.02.2021 01:50

Mathematics, 06.02.2021 01:50

Health, 06.02.2021 01:50



Mathematics, 06.02.2021 01:50







Arts, 06.02.2021 01:50

Biology, 06.02.2021 01:50

Chemistry, 06.02.2021 01:50


Mathematics, 06.02.2021 01:50




