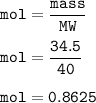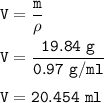Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 06:40
Three alkali metals in group 1 are a. calcium, strontium, barium b. boron, aluminum, gallium c. sodium, potassium, rubidium d. fluorine, iodine, chlorine
Answers: 1

Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3
You know the right answer?
How many milliliters of sodium metal, with a density of 0.97 g/mL, would be needed to produce 34.5 g...
Questions



Mathematics, 05.03.2021 19:30


Mathematics, 05.03.2021 19:30


Mathematics, 05.03.2021 19:30


Arts, 05.03.2021 19:30

History, 05.03.2021 19:30

Mathematics, 05.03.2021 19:30

Mathematics, 05.03.2021 19:30



Biology, 05.03.2021 19:30


History, 05.03.2021 19:30


Mathematics, 05.03.2021 19:30