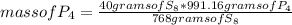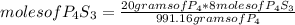Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2

Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1

Chemistry, 23.06.2019 00:30
How many moles of co2 are produced during the complete combustion of 3.6 moles of c2h6
Answers: 1
You know the right answer?
8P4 + 3S8 -> 8P4S3
The molar masses of the substances are as follows: P4 = 123.895g/mol, S8 = 25...
Questions

Mathematics, 19.01.2021 19:00

English, 19.01.2021 19:00

Mathematics, 19.01.2021 19:00



Biology, 19.01.2021 19:00

Mathematics, 19.01.2021 19:00



Mathematics, 19.01.2021 19:00

Mathematics, 19.01.2021 19:00



English, 19.01.2021 19:00

French, 19.01.2021 19:00





History, 19.01.2021 19:00