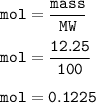Chemistry, 15.12.2020 14:00 royaltyy6533
A scientist completes a thermal decomposition reaction. 12.25g of calcium carbonate, CaCO3, is heated
and forms calcium oxide, Cao, and carbon dioxide, CO2.
a. Write a balanced chemical equation to demonstrate this reaction. Include state symbols.
b. Calculate the number of moles of calcium carbonate that is thermally decomposed in this
reaction.
Ok

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

Chemistry, 22.06.2019 21:20
The organs inside the body and how they function together
Answers: 3

Chemistry, 23.06.2019 01:00
Which statement is true regarding the diagram of circle p? the sum of y and z must be 2x. the sum of y and z must be x. the difference of z and y must be 2x. the difference of z and y must be x
Answers: 1
You know the right answer?
A scientist completes a thermal decomposition reaction. 12.25g of calcium carbonate, CaCO3, is heate...
Questions



Mathematics, 04.09.2019 16:10







Computers and Technology, 04.09.2019 16:10


Computers and Technology, 04.09.2019 16:10



English, 04.09.2019 16:10




Computers and Technology, 04.09.2019 16:10